Research
KEY WORDS | Pathophysiology of medically intractable epilepsy and its treatment | Mapping higher cortical functions/network and elucidating its functional alteration under pathological condition | Pathogenesis of movement disorders and its treatment | Collaborators
Our main goal is to solve so-called “clinical questions”, which have been raised in the daily clinical activity, and have remained unsolved yet.
Based on the concept of system neuroscience, by means of established and newly developed various methods, many clinical- and basic researches are conducted as follows.
We are carrying out multidisciplinary collaborative researches by joining the two research groups funded by grant-in-aid for scientific research on innovative areas (JSPS), namely, “Non-linear Neuro-oscillology: Towards Integrative Understanding of Human Nature” and “Understanding brain plasticity on body representations to promote their adaptive functions“.
KEY WORDS
General key words: epilepsy, epilepsy surgery, higher cortical function (motor control, praxis, language, semantic cognition, vision, will), Bereitscheftspotentials (BPs), cortico-cortical network, movement disorders, sleep disorders, autoimmune epilepsy, wideband EEG
Unique key words: ictal DC shifts, cortico-cortical evoked potentials (CCEP), cortical tremor, ictal apraxia, ictal paresis
1)Pathophysiology of medically intractable epilepsy and its treatment
a)Shaping presurgical evaluations for intractable epilepsy
Even in the 21st century, electroencephalography (EEG) remains essential in the diagnosis of “epileptogenicity”.Simultaneous recording of EEG and fMRI (EEG-fMRI) is a new technique that takes advantages of both modalities and complements each other to delineate both the cortical and subcortical structures related with epileptic activities.We for the first time introduced this technique to Japan to investigate the epileptic network and underlying pathophysiology in various types of epileptic syndromes such as praxis-induced epilepsy and hypothalamic hamartoma.
Epilepsy surgery has been established as an option for treatment of intractable partial epilepsy. The epileptogenic lesions, such as hippocampal sclerosis, cavernous angioma, and brain tumor, are the most common candidates for the one-stage surgery. It is still a challenge to localize the epileptic focus in ‘MRI-negative’ patients. We have extensively made multidisciplinary approaches with MEG, EEG-fMRI and FDG-PET to ‘visualize’ the epileptic focus in these MRInegative patients for possible treatment with epilepsy surgery.
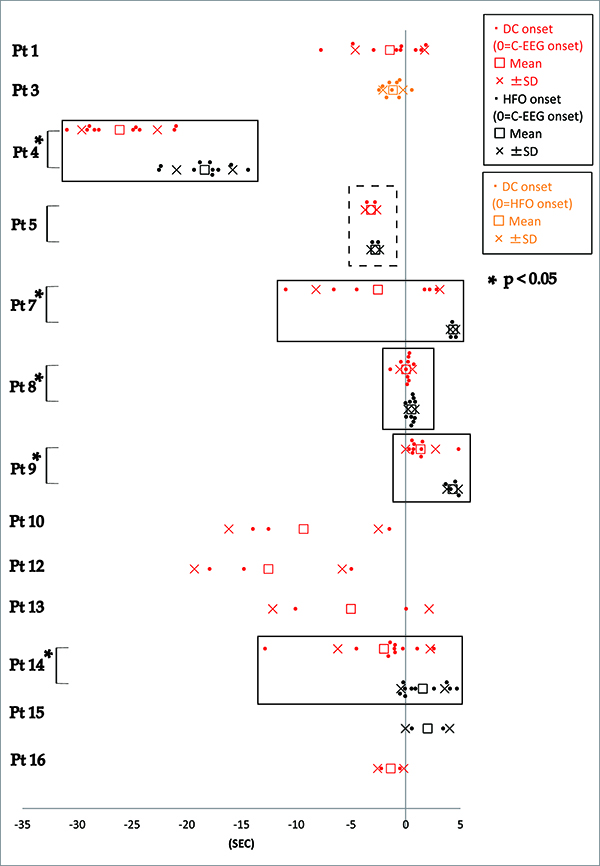
Especially in the MRI-negative cases, we occasionally need invasive evaluation with intracranial electrodes to precisely delineate the epileptic focus and map the eloquent cortices at and around the epileptic focus. In addition to the conventional frequency band (Berger rhythm: 0.3-70Hz), advancement in medical engineering has enabled us to record electrocorticogram with a broader frequency band (wideband EEG), ranging from direct current shifts (DC shifts) to high frequency oscillation (HFO). In our recent report, several features of ictal DC shifts and HFOs were extracted in 16 patients who underwent chronic intracranial recording below (Fig. 1) (Kanazawa et al., 2014).
1) Sensitivity and reproducibility: Ictal DC shifts were identified in 12 out of 16 patients. In these 12 patients, ictal DCshifts occurred in 71.3% of all seizures on average.
2) Temporal profile: Out of seven patients with ictal DC shifts and HFOs together, five patients showed DC shifts preceding HFOs with statistical significance.
3) Spatial profile: In 15 patients with conventional ictal pattern, 12 patients showed DC shifts and/or HFOs. Among them, DC shifts and HFOs were observed in more restricted area than conventional ictal pattern. Mean number of electrodes showing DC, HFO and conventional ictal pattern were 9.0±11.5, 10.4±8.4 and 16.3±17.2, respectively.In one patient, DC shifts were recorded from the depth electrode in the white matter, but HFOs were not recorded.
4) Relevance to pathological findings: DC shifts were identified in ① 75% (3/4) of glioma, ② 77.8% (7/9) of cortical dysplasia and ④ 50% (1/2) of hippocampal sclerosis. On the other hand, HFOs were in ① 50% (2/4), ② 44.4% (4/9),③ 50% (1/2).
We have been applying such a wideband EEG technology (ictal/interictal DC shift and HFO) to further localize theIII Activity reportResearch activities48core epileptogenic zone for epilepsy surgery.
An in vivo electrical tract tracing method by cortico-cortical evoked potentials (CCEPs) is an exciting new technique for presurgical cortical mapping developed in Cleveland Clinic andKyoto University. By means of subdural electrodes implanted for presurgical evaluation, electrical pulses(0.3-ms duration, frequency of 1 Hz, alternating polarity, 1‒10 mA) are directly delivered to the cortex,and CCEPs are obtained from adjacent and remote cortical regions by averaging the electrocorticogram time-locked to the stimulus onset. It promises to refine our understanding of surgical candidacy, first through a more precise and tailored evaluation of the seizure network in each individual patient,and second through greater understanding of the functional systems of the brain involved. Both are important for improving our ability to identify patients at high risk for poor surgical outcomes across multiple outcome measures.
b) Unraveling pathophysiology for treatment of epilepsy
As described above, EEG-fMRI could evaluate the whole brain including the subcortical structures.Using this technique, we have been studying the pathological basis of praxis-induced epilepsies and symptomatic epilepsy caused by hypothalamic hamartoma, a model of subcortical epilepsy and epileptic encephalopathy.
Recently the role of autoimmunity has been highlighted for a subset of encephalitis and epileptic seizures. Nowadays, high-resolution MRI can depict subtle structural changes in the medial temporal lobe, such as hippocampus and amygdala, which are often the main inflammatory foci in autoimmune limbic encephalitis. By means of comprehensive study such as video-EEG monitoring (VEEG), neuropsychological tests, FDG-PET and MRI,we have revealed the smoldering nature of the autoimmune limbic encephalitis (Fig. 2).
We have been utilizing multidisciplinary approach (VEEG, FDG-PET and 3T MRI) for offering tailor-made immunotherapy for patients with autoimmune epilepsy. Additionally, we reported that autoimmune mechanism could underlie in an epileptic patient with atypical clinical picture (triple pathology in a patient with parietal tumor) (Matsumoto et al., 2015).
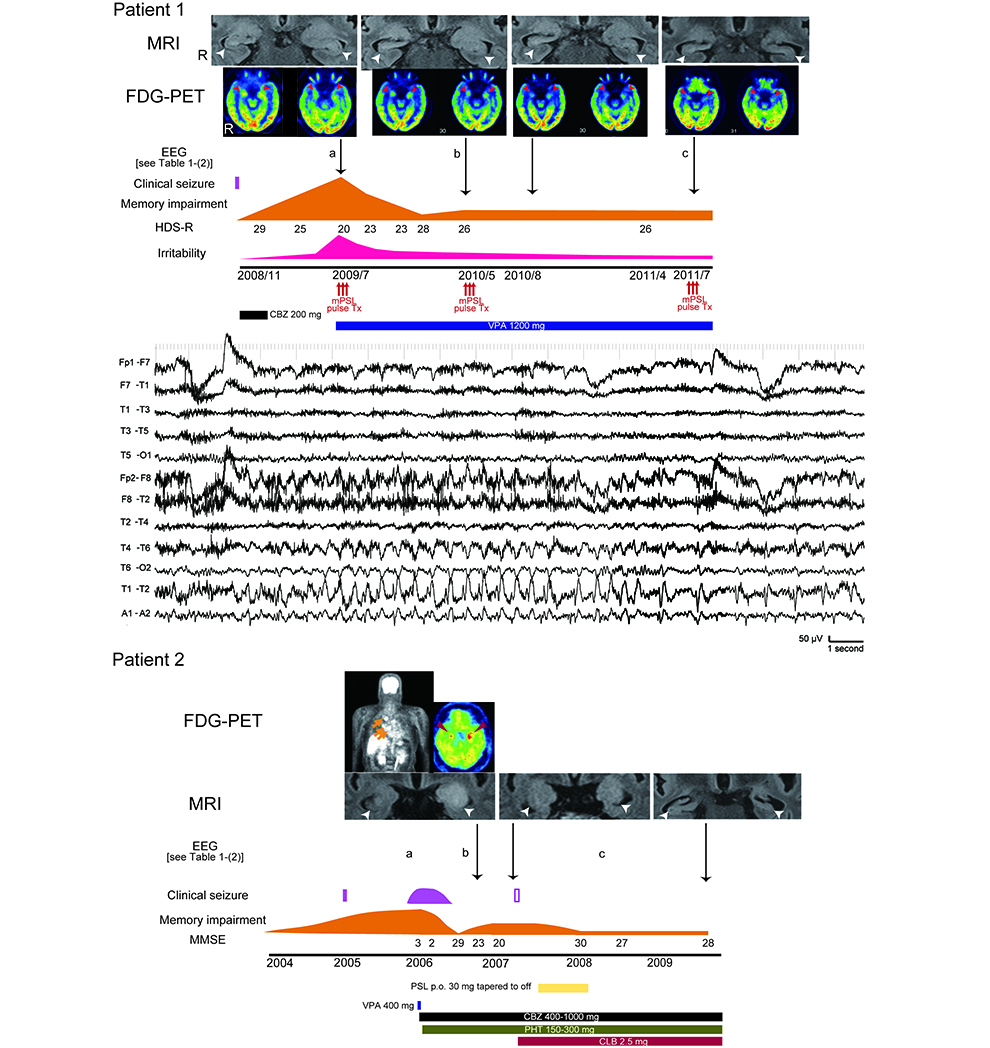
Figure 2.
A patient with autoimmune encephalitis (positive for anti-VGKC complex antibodies) in our institute.Imaging: Initial MRI and FDG-PET revealed abnormal intensity/swelling and hypermetabolism inthe bilateral mesial temporal lobes, respectively (arrow heads). Later, the bilateral mesial temporallobes had become atrophic on MRI and showed improved hypermetabolism on FDG-PET gradually(top).
Clinical course: the symptoms fluctuated and prolonged despite of infrequent clinical seizure(middle). Subclinical EEG seizure pattern: rhythmic activities evolve into lower frequency in the righttemporal area (bottom). The occurrence rate of EEG seizure patterns corresponded to activity ofencephalitis. (Kanazawa et al., 2014)
Interestingly, LGI1 antibody and LGI1 gene mutation present different phenotypes: LGI1 antibody could mediate autoimmune limbic encephalitis whereas LGI1 is the causative gene for autosomal dominant lateral temporal lobe epilepsy (ADLTE). We established Lgi1 heterozygous mutant rats (Lgi1L385R/+) as an animal model of ADLTE in close cooperation with the Institute of Laboratory Animals, and are currently promoting the project with Prof. Ono’s lab in Osaka University of Pharmaceutical Sciences.
In collaboration with the Department of Pharmacy in Kyoto University Hospital, we have extensively investigated the effect of the gene polymorphism to tailor dosage of antiepileptic medication, such as CYP2C19 polymorphism for efficacy of clobazam.
As an interventional neurophysiology, we have introduced neuro-feedback therapy for patients with intractable epilepsy. Using the DC-EEG machine, we have performed clinical trials in which the patients learn to regulate their slow cortical potential (SCP) and thereby decrease their cortical excitability. The neuro-feedback therapy is side-effect free and regarded as one of the promising alternative therapies.
We started conducting collaborative researches in epilepsy by means of iPS cells with Prof. Haruhisa Inoue (Center for iPS Cell Research and Application: CiRA).
We now try to elucidate the mechanism of vagus nerve stimulation for seizure reduction in collaboration with KinkiUniversity (Prof. Kato) and Hiroshima University (Prof. Iida).
c) Advanced EEG analysis
EEG now has a history of more than 80 years for evaluation of brain functions and diagnosis of brain diseases,ranging from brain death, coma to epilepsy. Medico-engineering collaboration between Prof. Shibasaki’s group (KyotoUniversity School of Medicine,
Neurology, Human Brain Research Center) and Prof. Nakamura’s group (Saga University,Faculty of Science and Engineering) has been done to develop the automatic EEG interpretation system, and it is currently under clinical investigation.
2) Mapping higher functions/network and elucidating its functional alteration under pathological condition
In epilepsy surgery, it is important to map cortical functions to preserve eloquent cortices in addition to the localization of the epileptic focus. Therefore, we need to perform comprehensive ‘system mapping’ to help neurosurgeons to make strategy of surgery for individual patients. We have made vigorous attempts at developing various techniques for mapping higher cortical functions (e.g., language, semantic cognition, motor control) and their network for clinical application.
Functional neuroimaging tells us if specific brain regions are active during certain tasks, but activation by itself does not demonstrate the necessity of those areas. In contrast, electrical cortical stimulation, a gold standard method since mid-20th century, can delineate the cortex responsible for a particular task by making functional impairment. The functional interference is temporary (~5s), discretely focal (~1cm2), and in sharp contrast to chronic stroke lesions those are relatively large and usually associated with cortical plastic compensation.
However, high frequency electrical stimulation often results in afterdischarges that delay functional mapping and harbor a risk of seizure induction. Recent technical advances have enabled us to record the cortical activities relevant to higher cortical functions with wideband EEG technology – from infraslow to high gamma activities. In our institute,in addition to the gold standard method of high frequency electrical stimulation, we perform comprehensive mapping of higher cortical functions by recording epicortical event-related potential, Bereitschaftspotential (BP) and high frequency oscillation/activity. Recently, we investigated the role of the basal temporal language area (BTLA)using a comprehensive approach (Shimotake et al., 2014). Discrete local field potentials were observed in BTLA while participants completed semantic tasks. Electrical stimulation to the area evoked semantic paraphasia and prolonged reaction time selectively during the semantic tasks. The study demonstrated that the BTLA is crucial for semantic processing especially among language functions (Fig. 3). Furthermore, high frequency electrical stimulation to the area and its adjacent areas sometimes induced mirth or laughter, which demonstrated that the area is also related to semantic processing of humor (Yamao et al., 2014).
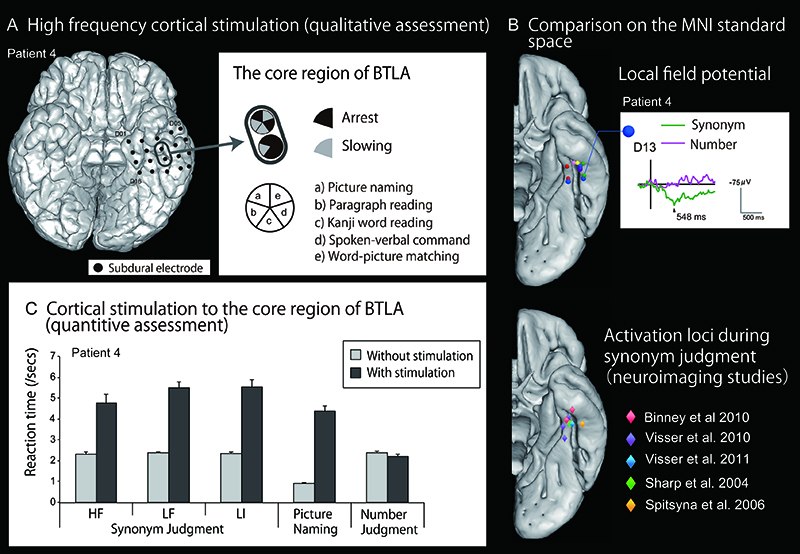
Figure 3.
Comprehensive mapping of the basal temporal language/semantic area A. Language mapping with conventional, qualitative high frequency cortical stimulation. Inthis representative case, high frequency cortical stimulation was delivered to the left basaltemporal lobe while the patient completed various language tasks. Impairment of the tasks(arrest or slowing) was investigated during stimulation. The black oval denotes the core regionof the basal temporal language areas (BTLA) defined by high frequency electrical stimulationand local field potentials obtained during naming. B. The six spheres in different colors onthe ventral view of the left hemisphere of standard MNI space shows the each core regionof BTLA in six patients (upper figure). Note their locations are consistent with activationareas in semantic functional neuroimaging studies of normal volunteers (lower figure). The representative local field potential (Patient 4) shows the clear positive activity for synonymjudgment (its processing supposedly requires access to semantic memory) while no apparentactivity for the control task of number size judgment. C. Quantitive assessment by corticalstimulation. Electrical cortical stimulation with weaker intensity was delivered to the same coreregion of BTLA, time-locked to the task. It produced significant slowing in synonym judgmentand picture naming tasks where semantic processing was required. In contrast, the reactiontime of the number size judgment task was not affected. Taken together with the local fieldpotential recording, the finding of quantitative electrical cortical stimulation suggest the activerole of BTLA in semantic processing. (modified from Shimotake et al., 2014 with permission)
We incorporate cortico-cortical evoked potentials (CCEPs) to probe inter-areal functional connectivity in order to perform ‘system mapping.’ For example, in close collaboration with the Department of Neurosurgery, we have applied CCEP intraoperatively to monitor the integrity of the dorsal language pathway. In this novel approach, we first define the anterior perisylvian language area (AL) according to presurgical anatomical/functional neuroimaging findings and CCEP connectivity patterns under general anesthesia. We then monitor the integrity of the language network by stimulating AL and by recording CCEPs from the posterior perisylvian language area consecutively during resection of the tumor in the vicinity of the arcuate fasciculus (Yamao et al., Hum Brain Mapp 2014, selected as theEditor’s Choice Award 2014). Furthermore, by gathering data of cortical functions and networks from many patients in various physiological and pathological states and analyzing them as a group, we attempt to feedback this valuable information into the system neuroscience by providing functional/connectivity references for non-invasive researches.This year, by comparing neural oscillations (high frequency activities, HFA >80 Hz) induced either by direct singlepulse cortical stimulation or peripheral median nerve stimulation, we elucidated that different propagation modes and/or different terminal layers determine HFA frequency bands (Kobayashi et al., 2015).
We have demonstrated the central mechanisms and functional alteration under pathological condition relevant to i) the motor control (conflict processing, negative motor phenomena, praxis and reaching), ii) language (dorsal and ventral language networks with emphasis on semantic cognition) and ⅲ) visual functions (retinotopic mapping by functional MRI), combined with non-invasive evaluations (functional MRI, diffusion tractography, MEG,neuropsychology). Additionally, we are now tackling with decoding of complex neural signals during various tasks in cooperation with seasoned researchers in and out of the Kyoto University (Graduate School of Informatics, Dr. SatoshiTsujimoto; Advanced Telecommunications Research Institute International, Dr. Rieko Osu; School of PsychologicalSciences, the University of Manchester, Prof. Matthew Lambon-Ralph). Finally, we are pleased to announce that our 4-year activity of international/domestic neural oscillation conference led to launching the national research group of “Neuro-Oscillology” which is funded by Grant-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology (MEXT). Under the leadership of Prof. Atsushi Nambu at theNational Institute for Physiological Sciences, Professor Ikeda will manage the research project A01 on “the DirectRecording of Human Neural Oscillations”.
3)Pathogenesis of movement disorders and its treatment
We have investigated movement disorders, mainly myoclonus and myoclonus epilepsy, by way of epidemiological,genetical and electrophysiological methods. BAFME (benign adult onset familial myoclonus epilepsy) has been investigated mainly in Japan and European countries for 20 years. The clinical pictures are as follows: i) adult onset, ii) autosomal dominant (unknown causative gene), iii) cortical (myoclonic) tremor (tremulous myoclonus), iv)infrequent generalized seizure, v) cortical reflex myoclonus disclosed by electrophysiological study. We have also been studying BAFME since it was first reported in 1990. As its name suggests, BAFME was considered to present no progression and good prognosis. However, cortical myoclonic tremor has been proved to worsen with aging.Recently, we demonstrated slow progression of the disease, based on the electrophysiological evidence. Namely,the amplitude of somatosensory evoked potential, reflecting the cortical excitability in the primary sensori-motor cortices, more exaggerated with aging in BAFME patients than normal volunteers. We also demonstrated clinical anticipation in BAFME, in which the onset of generalized seizure and cortical (myoclonic) tremor became earlier in the next generation. The anticipation in BAFME was more apparent in patients with maternal transmission. These findings would be helpful to search a causative gene of BAFME. In addition, the nationwide questionnaire for neurologists and epileptologists in Japan revealed that BAFME patients were found diffusely without regional accumulation (Fig. 4:right).
Unverricht-Lundborg disease (ULD) is the most common form of progressive myoclonus epilepsy syndrome (PME) in the world, but mainly reported from Baltic and Mediterranean region. The symptoms consist of epileptic seizure,myoclonus, ataxia and cognitive impairment and are gradually deteriorated. We reported ULD cases in Japan, in someof which the development of symptoms and increase of SEP amplitude became very subtle for long-term follow-up(Fig. 5) (Kobayashi et al., 2014). Similar to BAFME, PME including ULD also presented no regional clustering in Japan (Fig.4: left).
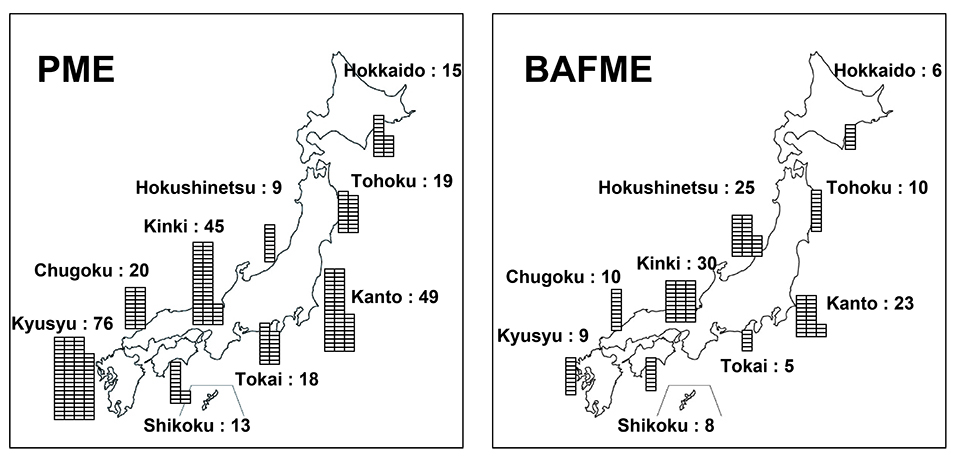
Figure 4.
Regional distribution of PME and BAFME in Japan investigated by the nationwide questionnaire. There is no regional clustering in both diseases.
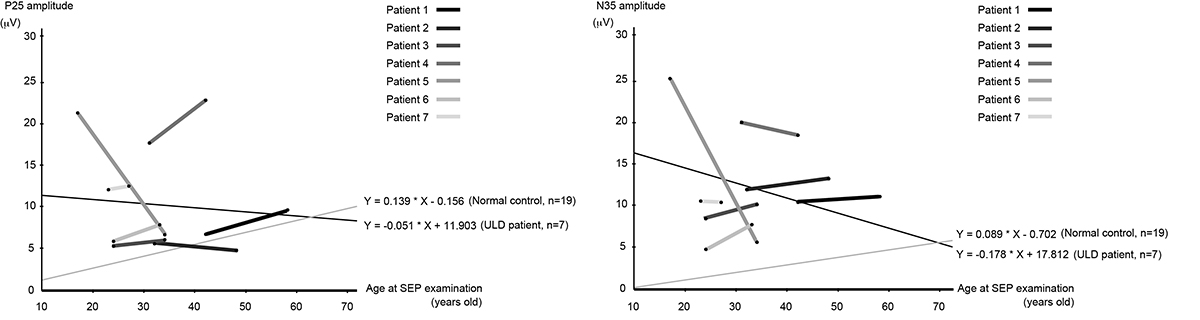
Figure 5.
Correlation between the age at SEP recording and its amplitudes in 7 ULD patients. Values of P25 and N35, and the gradients of their temporal changes drawn by calculating the averaged value of the ages and amplitudes between previous and present SEP recordings (black lines) in 7 patients are compared with linear regression curves of 19 healthy subjects (gray lines). P25 and N35 amplitudes were larger in ULD patients. As for the components associated with giant SEP (i.e., P25 and N35), 7 ULD patients showed a tendency of slight decrease in amplitude during the follow-up period, and the degree of its tendency was even lower than that of healthy subjects.
Collaborators
We have been collaborating closely with the Departments that officially support our department. Other collaborators are listed below.
[Overseas]
Dr. Stéphanie Baulac, Ph.D.
Affiliation: Institut du Cerveau et de la Moelle épinière (ICM), Epilepsy Unit
Position: Research Director
Dr. Christophe Bernard, Ph.D.
Affiliation: INS – Institut de Neurosciences des Systèmes, UMR INSERM 1106, Aix-Marseille Université
Position: Team leader
Dr. Marco Catani, M.D., Ph.D.
Affiliation: Natbrain lab, Department of Forensic and Neurod evelopmental Sciences, Institute of Psychiatry, King’s
College London
Position: Clinical Senior Lecturer and Honorary Consultant Psychiatrist
Prof. Nathan Earl Crone, M.D.
Affiliation: Department of Neurology, Johns Hopkins University School of Medicine
Position: Professor
Dr. Marco De Curtis, M.D.
Affiliation:Fondazione IRCCS Istituto Neurologico Carlo Besta
Position:Head of Epileptology and Experimental Neurophysiology Unit, Head of Pre-clinical Neuroscience Laboratories
Prof. Mattew A. Lambon-Ralph, FRSLT (hons), FBPsS
Affiliation: School of Psychological Sciences, University of Manchester
Position: Professor of Cognitive Neuroscience & Associate Vice-President (Research)
Dr. Dileep R. Nair, M.D.
Affiliation: Epilepsy Center, Cleveland Clinic
Position: The Section Head of Adult Epilepsy and Director of Intraoperative Neurophysiologic monitoring
Prof. Angela Vincent, Ph.D.
Affiliation: University of Oxford
Position: Emeritus professor
[Domestic]
Dr. Koji Iida, M.D., Ph.D.
Affiliation: Department of Neurosurgery, Hiroshima University Hospital
Position: Lecturer
Prof. Hiroshi Imamizu, Ph.D.
Affiliation:Department of Psychology, The University of Tokyo
Position:Professor
Dr. Yushi Inoue, M.D., Ph.D.
Affiliation: Shizuoka Institute of Epilepsy and Neurological Disorders, National Epilepsy Center, Department of Clinical
Research
Position: Hospital director
Prof. Shigeki Kameyama, M.D., Ph.D.
Affiliation: Nishi-Niigata Chuo National Hospital
Position: Honorary hospital director
Prof. Amami Kato, M.D., Ph.D.
Affiliation: Department of Neurosurgery, Kinki University Hospital
Position: Professor
Prof. Takashi Nagamine
Affiliation: Department of Systems Neuroscience, Sapporo Medical University School of Medicine
Position: Professor
Dr. Eiichi Naito, Ph.D.
Affiliation:National Institute of Information and Communications Technology (NICT) Center for Information and Neural Networks
Position:Research Manager
Prof. Masatoshi Nakamura, Ph.D.
Affiliation: Research Institute of Systems Control, Institute for Advanced Research and Education, Saga University
Position: Emeritus professor
Prof. Shigeto Nishida, Ph.D.
Affiliation: Department of Information and Communication Engineering, Faculty of Information Engineering, Fukuoka
Institute of Technology
Position: Professor
Dr. Teiichi Onuma, M.D., Ph.D.
Affiliation: Musashino Kokubunji Clinic
Position: Honorary hospital director
Dr. Rieko Osu, Ph.D.
Affiliation: Department of Motor Control and Rehabilitation, ATR Conputational neuroscience Labs
Position: Department Head
Dr. Satoru Saito, Ph.D.
Affiliation: Division of Cognitive Psychology in Education, Kyoto University Graduate School of Education
Position: Associate Professor
Dr. Takenao Sugi, Ph.D.
Affiliation: Institute of Ocean Energy, Saga University
Position: Associate professor
Prof. Shoji Tsuji, M.D., Ph.D.
Affiliation: Department of Neurology, The University of Tokyo Hospital
Position: Professor
Satoshi Tsujimoto, Ph.D.
Affiliation: Department of Intelligence Science and Technology Graduate School of Informatics, Kyoto University
Position: Associate professor
Dr. Hiroki Yamamoto, Ph.D.
Affiliation: Graduate School of Human and Environmental Studies, Kyoto University
Position: Assistant professor
Dr. Ikuko Yano, Ph.D.
Affiliation: Department of Pharmacy, Kyoto University Hospital
Position: Deputy director of pharmacy/Associate professor
(Listed in the alphabetical order of their family names)